
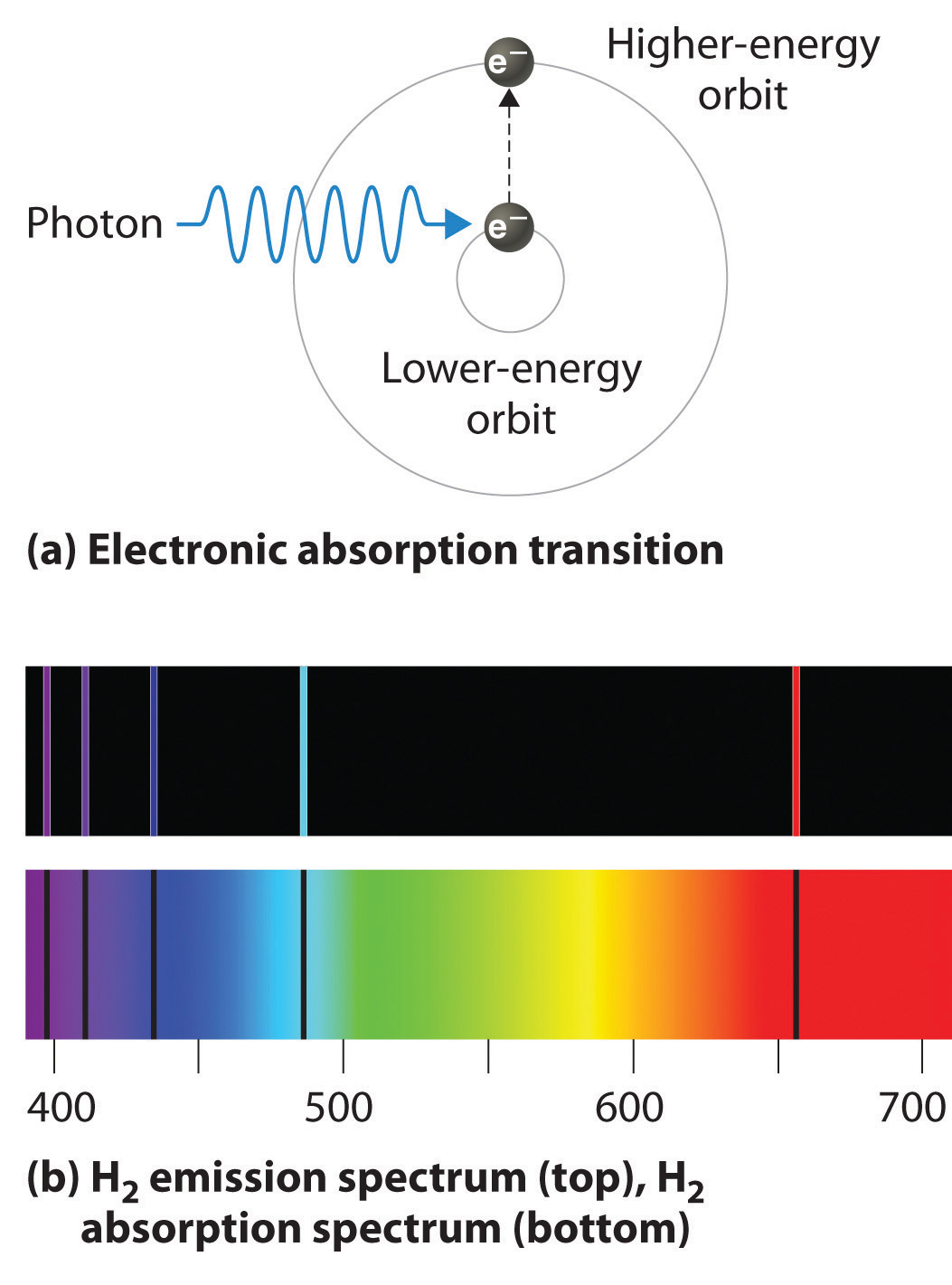
Other elements may need hotter flames to produce measurable spectra.Īnalysts use special techniques to properly interpret the results of flame analysis. Those that produce a measurable spectrum when subjected to flame include, but are not limited to: lithium, sodium, potassium, rubidium, cesium, magnesium, calcium, strontium, barium, zinc, and cadmium. Standard or Bunsen burner based flame tests do not work on all elements. Longer wavelengths represent red light, shorter wavelengths in the visible spectrum represent blue and violet light. Reception of light (photons) varying in frequency and wavelength is interpreted as color. Visible or color spectrum -That portion of the electromagnetic spectrum to which the human eye is sensitive. Spectrum -A display of the intensity of radiation versus wavelength. Massless, photons - because they are light -travel at the speed of light (c) after emission from an excited atom. Photon -The boson or carrier particle of light (electromagnetic waves).

To prevent this obscuration, the wire to be coated with the unknown sample is usually dipped in hydrochloric acid and subjected to flame to remove the volatile impurities and sodium. The yellow color of sodium, for example, can be so intense that it overwhelms other colors. Highly volatile elements (chlorides) produce intense colors. The unknown sample subjected to flame analysis is either sprayed into the flame or placed on a thin wire that is then introduced into the flame. Quantitative tests measure the amounts or proportions of the components in a reaction or substance. A qualitative chemical analysis is designed to identify the components of a substance or mixture. Qualitative testingįlame analysis is a qualitative test, not a quantitative test. The mathematically related frequencies and wavelengths of the photons emitted are characteristic for each element and this is the physical basis of the uniqueness of spectral fingerprints. Bunsen determined that the spectral patterns of elements that emitted light when subjected to flame analysis differed because each pattern represented limited portions of the total possible spectrum.įlame analysis or atomic emission spectroscopy is based on the physical and chemical principle that atoms -after being heated by flame -return to their normal energy state by giving off the excess energy in the form of photons of light. To examine the spectra of elements, Bunsen used a simple apparatus that consisted of a prism, slits, and a magnifying glass or photosensitive film. Often termed “spectral fingerprints, ” the color of the flame and its spectral distribution of component colors is unique for each element. This temperature is sufficient to cause the emission of light from certain elements. When air is admitted at the base of a Bunsen burner it mixes with hydrocarbon gas to produce a very hot flame at approximately 3,272 ☏ (1,800 ☌).

Analysis of emission spectraīunsen examined the spectra the colors of light emitted when a substance was subjected to intense flame. Using techniques pioneered by Bunsen, scientists have since been able to determine the chemical composition of a variety of substances ranging from bioorganic debris to the composition of the stars. Bunsen ’s techniques also enabled his discovery of the elements cesium and rubidium.īunsen ’s fundamental observation that flamed elements emit light only at specific wavelengths, and that every element produced a characteristic spectra, paved the way for the subsequent development of quantum theory by German physicist Maxwell Planck (1858 –1947), Danish physicist Niels Bohr (1885-1962), and others. Working with Gustav Kirchhoff (1824 –1887), Bunsen helped to establish the principles and techniques of spectro-scopy. German chemist Robert Bunsen ’s (1811 –1999) invention of the Bunsen burner -a tool now commonly used in chemistry laboratories -also spurred the development of flame analysis. Allowing analysis of the light (photons) from excited atoms, flame analysis is a form of atomic emissionspectroscopy (AES).


 0 kommentar(er)
0 kommentar(er)
